orange book pharmacy definition
FDA has draft guidance explaining that certain currently marketed drug ingredients were marketed before current FDA legislation. Get emails about this page.
Orange Book Approved Drug Products with Therapeutic Equivalence Evaluations Brand-generic therapeutic equivalencies Found in accessdatafdagov.
. The Orange Book Introduction. Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use. Acute A condition with a fast onset time severe effect and short course of duration3.
Enforce State Pharmacy Boards Oversight of Patient Counseling Laws. Orange-Book-Standard issued in 2009 by the German Federal Court of Justice on the interaction between patent law and standards. The Orange Book formally titled Approved Drug Products With Therapeutic Equivalence Evaluations is a comprehensive list of approved drug products published by the FDA.
First published in 1971 the original Orange Guide contained British Good Manufacturing Practice and was entitled Guide to Good Pharmaceutical Manufacturing Practice. R i s k r e. 2 approved over-the-counter OTC drug products for those drugs that.
G o v e r n a n c e and L e a d e r s I n te g ra o n h i p C o l a b or ti o n Information Insight Insight Information Communication. Orange Book a local area networking protocol based on the Cambridge Ring and one of the UK Coloured Book protocols. Office of Generic Drugs Policy Center for Drug Evaluation Research US.
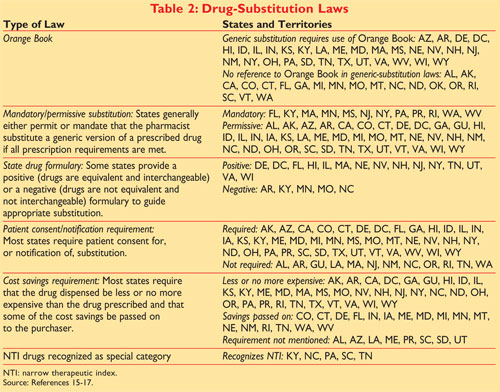
The orange book consist of five main sections. It is widely accepted as the authoritative source for determining therapeutic equivalence among multisource drug products. The full publication title is Approved Drug Products with Therapeutic Equivalence Evaluations but it is commonly known as the Orange Book.
The Orange Book is composed of four parts. First if you have the trade name search the Electronic Orange Books Rx or OTC section using the Proprietary Name search. Adverse reaction An undesired or negative response to a medication or drug-drug interaction2.
Food and Drug Administration. The FDAs Orange Book identifies approved drug products. The orange book is a list of generic drugs approved by FDA.
Not much more than 30 pages in length this voluntary guide was an aid to manufacturers to understand the needs of the regulatory authoritys requirements for the manufacture of. Updated with Orange Book. The Orange book has been revised.
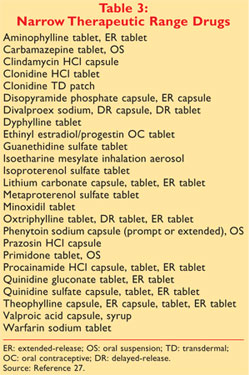
PCS - Dr Z. 1 approved prescription drug products with therapeutic equivalence evaluations. The Orange Book is a reference source that gives insight on whether or not two drugs have Therapeutic Equivalences.
Then use the Ingredient Search for. The Orange Book does not include drugs. The Orange Book Risk Management Principles.
The Office of Inspector General. Rucha Pathak Roll No. CDR Kendra Stewart RPh PharmD.
Before understanding different drug ratings it is necessary. The Orange Book is a compendium of significant unimplemented nonmonetary recommendations for improving departmental operations. Food and Drug AdministrationFDA has approved as both safe and effective.
Sets with similar terms. Food and Drug Administration 10001 New Hampshire Ave Hillandale Bldg 4th Floor Silver Spring MD 20993 -0002 Phone. The orange book is published annually and the 2015 edition is 35th edition of orange book1 It is freely available for.
Approved Drug Products with Therapeutic Equivalence Evaluations. AC prescription Before a meal3. This determines the ingredient s.
Sponsors using these products should consult FDA about the need for an IND. Use of unapproved drugs require complete CMC information depending on the nature of the study. FDA orange book The official name of FDAs orange book is Approved Drug Products with Therapeutic Equivalence Evaluations.
Handbook of Directives and Permitted Conventions for the English Bridge Union. Rules and Guidance for Pharmaceutical Manufacturers and Distributors commonly known as the Orange Guide brings together all the main European and UK directives regulations and legislation relating to the manufacture and distribution of medicines. Originally this book was published in October 1980 with orange cover and thus the name orange book.
The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution. Admixture Two or more drugs blended or mixed to create a desired substance or solution3. Generic Drugs and The Orange Book.
An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum. Although it is commonly called the Orange Book its formal name is Approved Drug Products with Therapeutic Equivalence Evaluations. 1 Generic substitution laws are state specific and many require use of the.
The Orange Book is a list of drugs and pharmaceuticals that the US. 65 Strengthen FDA. Basics in drug approval process with reference to the Orange Book Presented by.
The 2022 edition of Rules and Guidance for Pharmaceutical Manufacturers and Distributors the Orange Guide is now available through. FDAs Approved Drug Products with Therapeutic Equivalence Evaluations Orange Book identifies drug products approved on the basis of safety and effectiveness.

The Introduction Of An Orange Book

The Introduction Of An Orange Book

Investigational New Drug Orange Book Understanding On 505 B 2 A

The Introduction Of An Orange Book
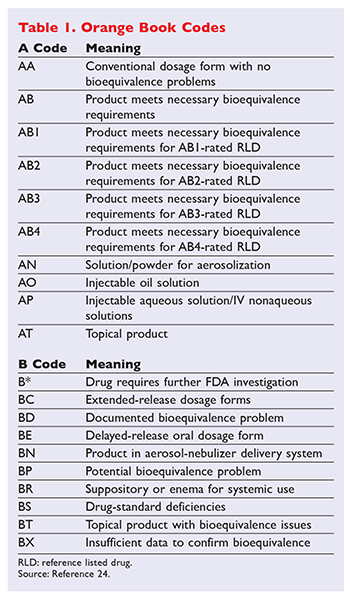
Insights Into Effective Generic Substitution

Orange Book And Its Applications Legal Advantage

The Introduction Of An Orange Book
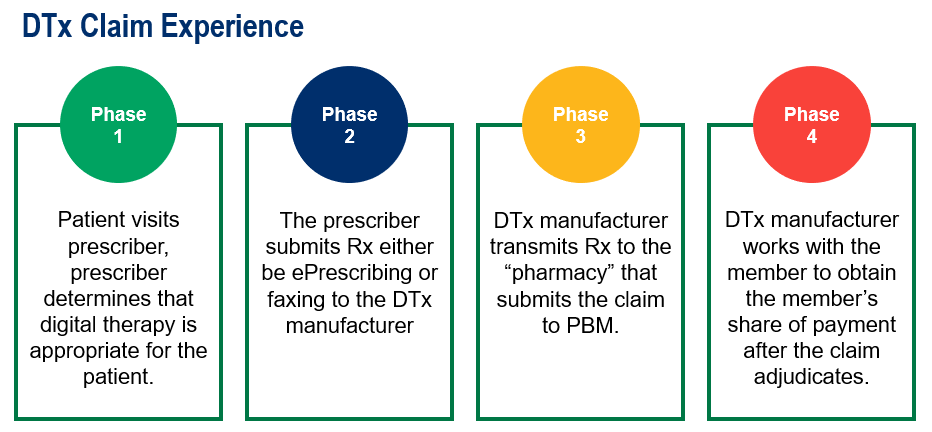
What Are Digital Therapeutics Rxbenefits
Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt

The Introduction Of An Orange Book
Appendix A Medical Terminology And Abbreviations In Manual For Pharmacy Technicians
/doctor-826e0c116cd549d98e327f1184c622d9.jpg)